Antibiotic Dosages Where Susceptibility is Reported as (Sensitive - increased dose required).
From now on (Sensitive - increased dose required) will replace the old (susceptible - increased resistance) antibiotic sensitivity category.
New categories for antibiotic susceptibility testing:
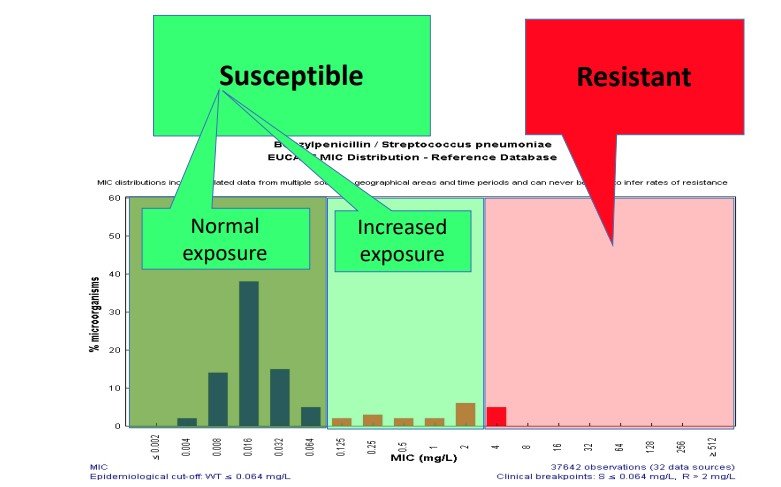
- Sensitive: A microorganism is categorised as (Sensitive), when there is a high likelihood of therapeutic success using a standard dosing regimen of the agent.
- Sensitive - increased dose required: A microorganism is categorised as (Sensitive - increased dose required) when there is a high likelihood of therapeutic success because exposure to the agent is increased by adjusting the dosing regimen or by its concentration at the site of infection.
- Resistant: A microorganism is categorised as (Resistant) when there is a high likelihood of therapeutic failure even when there is increased exposure.
The change to (Sensitive - increased dose required) recognises that for certain bug / drug combinations an increased dose of an antibiotic should be given to assure the organisms are exposed to adequate concentrations of the antibiotic at the site of infection.
Figure 1- Illustration of new categories for antibiotic susceptibility testing. Taken from Guidance to Clinical Colleagues EUCAST
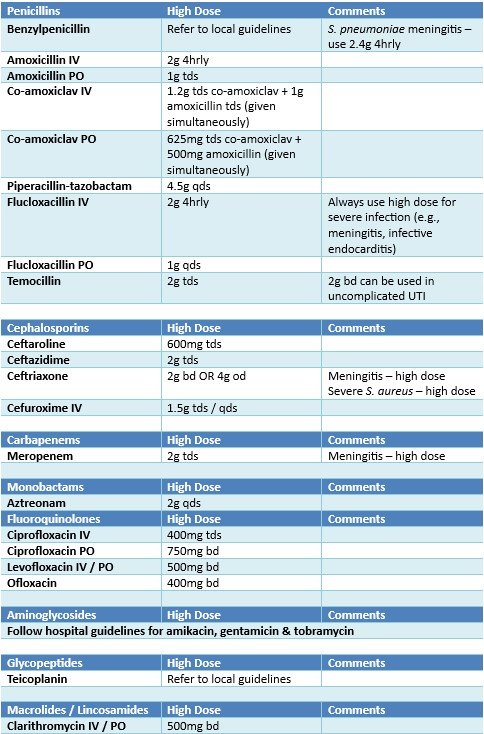
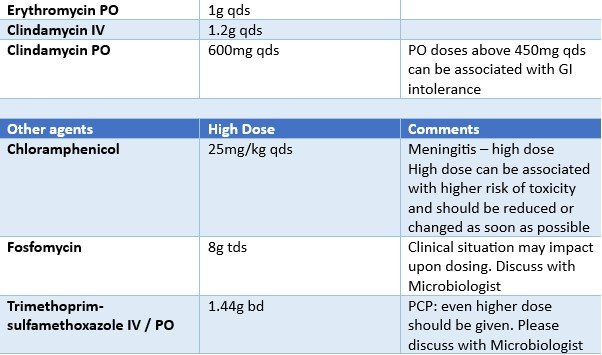
Local guidelines will indicate the higher dose as standard for some instances.
Please always follow local guidelines in the first instance for dosing advice, referring to the table below only when an antibiotic has been reported as (Sensitive - increased dose required).
Increased doses should not be used routinely in more severe infections or in immunocompromised patients unless specifically outlined in local antibiotic guidelines.
For some bug/drug combinations, the dosing for PO and IV administration may be reported separately so please be sure which route of administration is to be used.
The following table outlines what increased adult doses for relevant antibiotics are. Recommendations apply to systemic antibiotics only and do not relate to topical doses.
For paediatric dosing please contact the duty or on-call Consultant Medical Microbiologist for advice.
Please continue to consider renal and hepatic dysfunction prescribing antibiotics at increased doses and adjust accordingly. Please refer to the BNF, ward Pharmacist or discuss with Consultant Medical Microbiologist if there are concerns.
This list is not exhaustive and if there are any queries contact the Consultant Medical Microbiologist
For further explanation of the rationale behind the breakpoints see: Guidance to Clinical Colleagues EUCAST
References:
- European Society of Clinical Microbiology and Infectious Diseases. EUCAST ClinicalBreakpoints and dosing of antibiotics. Version 12. European Committee on Antimicrobial Susceptibility Testing, Jan 2022. https://eucast.org/clinical_breakpoints
- Scottish Antimicrobial Prescribing Group. Changes to antibiotic susceptibility reportingmicrobiology laboratories. January 2022 https://www.sapg.scot/guidance-qi-tools/antimicrobial-specific-guidance/eucast-changes/
- Infectious Diseases Society of America (IDSA) Guidance on the Treatment of Antimicrobial-Resistant Gram-Negative Infections: Version 2.0. Published 03/31/2022. https://www.idsociety.org/practice-guidelines/amr-guidance-2.0/
- European Society of Clinical Microbiology and Infectious Diseases. Breakpoints for temocillin. European Committee on Antimicrobial Susceptibility Testing, Apr 2020