Ganciclovir
Department of microbiology.
Notes:
- The information given here is intended for use by healthcare professionals. Please see Lab Tests Online-UK for more general advice, links and background.
- Assays of value in patients with renal failure and to monitor oral absorption, particularly in patient receiving valganciclovir.
- Advanced warning of referral is not normally required, during our core working hours (Monday to Friday 9am to 5.15pm).
- Please contact the laboratory to discuss the possibility of this assay being performed on Saturdays, Sundays and bank holidays.
Sample required
Approx. 1-2mL of separated serum (the minimum acceptable is 100µL). Samples may be sent by post, but transport time must be less than 7 days. Samples should be kept at 4°C if there is a delay in sending. Please contact the laboratory if in doubt as sample integrity may be affected.
Test information required
- patient name, sex and age
- laboratory number
- dosage, frequency and timing of samples
- clinical summary
- address for report
- phone or fax number for report or email address for an electronic copy
- contact name
- appropriate hazard warnings
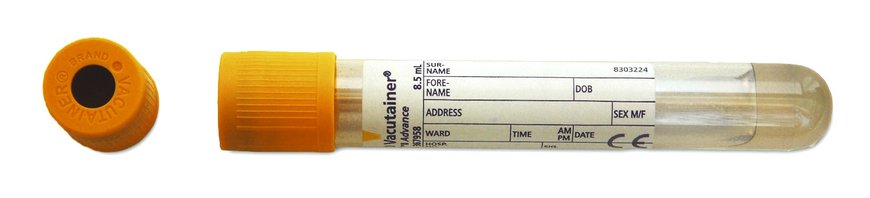
Sample to be collected in a gold topped serum tube.
Timing of samples
We recommend a pre-dose sample and a post-dose sample, taken either 1 hour after the end of IV administration or 2 hours after oral administration.
Results interpretation
Results will be made available for reporting in Sunrise as soon as they are reported back to Microbiology by the Reference Laboratory.
Reference ranges (target levels)
- Pre dose 0.5-1.0 mg/L – for prophylaxis
- Pre dose 1.0-2.0 mg/L – for treatment
- Post dose 7-9 mg/L (ganciclovir)
- Post dose 5-7 mg/L (valganciclovir)
Turnaround time
Less than 3 days.
Re-assay interval
4 to 8 days, assuming initial results are within expected range.