Respiratory virus PCR swabs, including COVID-19 PCR
Microbiology
Requesting and what to tell us
Please provide relevant clinical details and a date of onset.
Background
- The information given here is intended for use by healthcare professionals. Please see Lab Tests Online-UK for more general advice, links and background
- Used to diagnose respiratory viral illness from influenza (all types including H1N1), parainfluenza 1 -4, RSV, human metapneumovirus, adenovirus, Rhino/enterovirus and COVID-19.
- If the following are suspected please discuss with the Consultant Microbiologist and see the relevant PHE guidance:
Please check the current guidance for COVID-19 testing before sending samples for COVID PCR. See: Action Card: Guidance for testing.
For patients that meet the criteria for COVID-19 and/or Influenza like illness (ILI), and require respiratory PCR testing, please swab the patients throat and then using the same stick, swab the nasal passage.
Please specify on the request form what testing is required.
The laboratory has different PCR platforms available for respiratory virus testing with varying numbers of viral targets. The most appropriate platform is chosen in accordance with the clinical details provided. Therefore:
- For Influenza like illness where concern is a possible non-influenza, non-RSV cause, please discuss testing with Consultant Microbiologist prior to sending samples. Haematology, Oncology and neonatal patients will be tested against the full respiratory panel routinely.
- If rapid PCR testing for either Influenza or COVID-19 is required, please indicate as such on the request form and advise the laboratory where possible. This service is only available during the core laboratory hours.
Results for all PCR tests will be uploaded and available through the Sunrise EPR results system throughout the day.
Please do not call the Microbiology department (laboratory or CMM) for results.
See the UKSHA guidance on the use of antiviral agents for the treatment and prophylaxis of seasonal influenza: Guidance on use of antiviral agents for the treatment and prophylaxis of seasonal influenza (publishing.service.gov.uk)
Sample requirements for general Respiratory (Including COVID 19) PCR
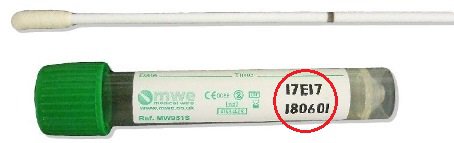
Viral swab
Check swab expiry date before use - (Date is in YYMMDD format)
Optimally a combined swab should be taken from the throat and nose, placing each swab into its transport tube following swabbing.
Nose only and Throat only swabs can be processed, but this could potentially lead to a reduced sensitivity.
Storage/transport
Transport as soon as possible at ambient temperature
If transport is delayed, samples may be stored at room temperature for up to 24 hours
Turnaround time
Samples may be tested locally or sent to a Regional Reference Centre
Turnaround times will vary according to the seasonal workload. During winter months and outbreaks - up to 3 days.